Know the difference between cleaning, disinfecting, and sanitizing
Cleaning removes germs, dirt, and impurities from surfaces or objects. Cleaning works by using soap (or detergent) and water to physically remove germs from surfaces. This process does not necessarily kill germs, but by removing them, it lowers their numbers and the risk of spreading infection.
Disinfecting kills germs on surfaces or objects. Disinfecting works by using chemicals to kill germs on surfaces or objects. This process does not necessarily clean dirty surfaces or remove germs, but by killing germs on a surface after cleaning, it can further lower the risk of spreading infection.
Sanitizing lowers the number of germs on surfaces or objects to a safe level, as judged by public health standards or requirements. This process works by either cleaning or disinfecting surfaces or objects to lower the risk of spreading infection.
Read Full Article
How to Make Your Own Disinfectant Bleach Solution
Bleach Sanitizing and Disinfection Solution Preparation Instructions
This is not a substitute for official rules and regulations; this document is only to assist you with solution preparation. Please contact your local health authority for office rules and regulations.
"Household bleach" means bleach sold in concentrations that are intended for household use, and not industrial applications. Household bleach is sold in retail stores at strengths of 5.25% sodium hypochlorite (regular strength bleach) solution and 6.00% sodium hypochlorite (ultra-strength bleach) solution.
Bleach to Water Mixing Ration
Contentration (%) of sodium hypochlorite can be found on the bleach container label
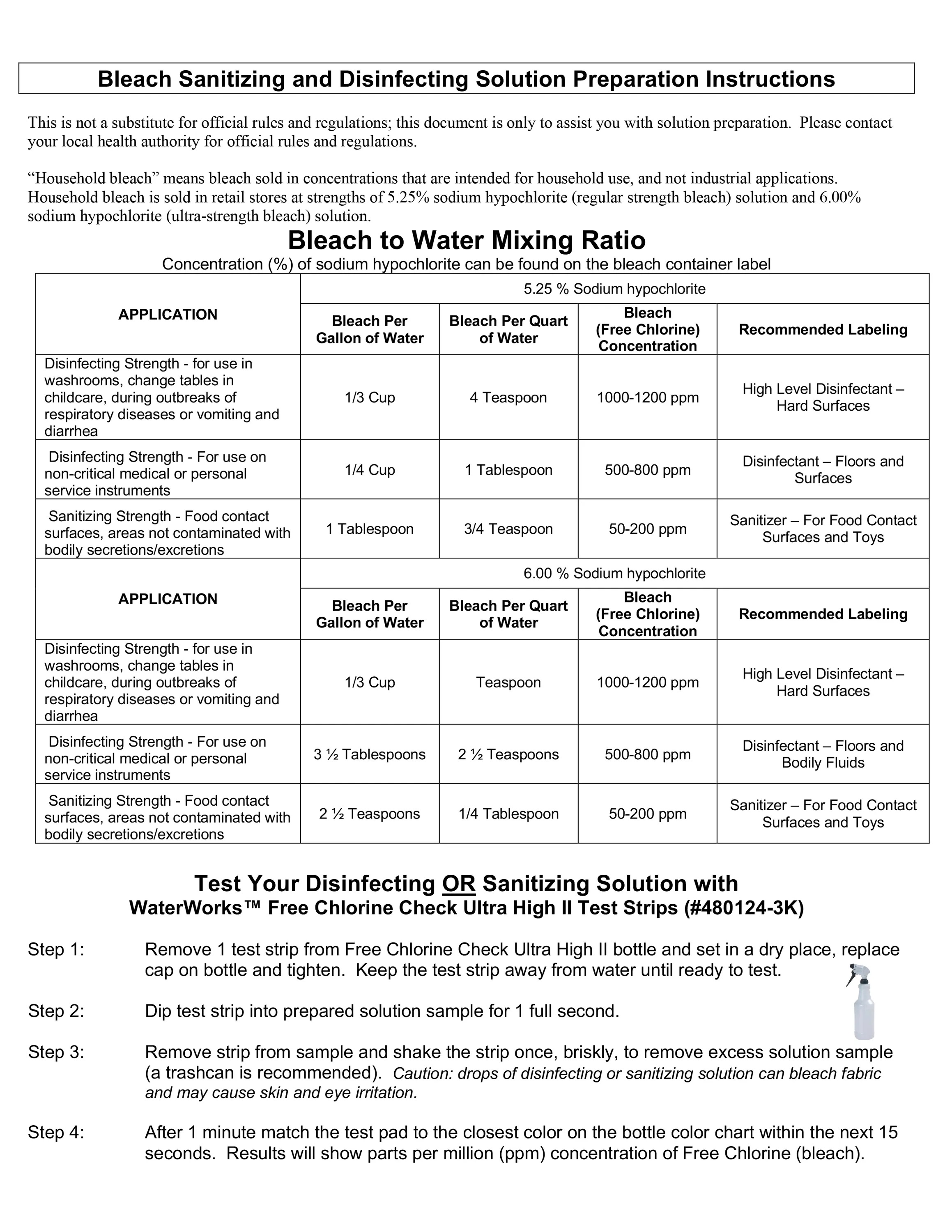
| Application |
5.25 % Sodium hypochlorite |
| Bleach per Gallon of Water |
Bleach per Quart of Water |
Bleach (Free Chlorine) concentration |
recommended Labelling |
| Disinfection Strength - for use in washrooms, change tables in childcare, during outbreaks of respiratory diseases or vomiting and diarrhea |
⅓ Cup |
4 Teaspoons |
1000-1200 ppm |
High Level Disinfectant - Hard Surfaces |
| Disinfection Strength - for use on non-critical medical or personal service instruments |
¼ Cup |
1 Tablespoon |
500-800 ppm |
Sanitizer - Floors and Surfaces |
| Sanitizing Strength - food contact surfaces, areas not contaminated with bodily secretions/excretions |
1 Tablespoons |
¾ Teaspoons |
50-200 ppm |
Sanitizer - For Food Contact Surfaces and Toys |
| Application |
6.00 % Sodium hypochlorite |
| Bleach Per Gallon of Water |
Bleach Per Quart of Water |
Bleach (Free Chlorine) Concentration |
Recommended Labelling |
| Disinfection Strength - for use in washrooms, change tables in childcare, during outbreaks of respiratory diseases or vomiting and diarrhea |
⅓ Cup |
1 Teaspoon |
1000-1200 ppm |
High Level Disinfectant - Hard Surfaces |
| Disinfection Strength - for use on non-critical medical or personal service instruments |
3 ½ Cups |
2 ½ Tablespoons |
500-800 ppm |
Sanitizer - Floors and Surfaces |
| Sanitizing Strength - food contact surfaces, areas not contaminated with bodily secretions/excretions |
2 ½ Tablespoons |
¼ Tablespoon |
50-200 ppm |
Sanitizer - For Food Contact Surfaces and Toys |
Test Your Disinfecting OR Sanitizing Solution with
WaterWorks™ Free Chlorine Check Ultra High II Test Strips (#480124-3K)
Step 1: Remove 1 Test strip from Free Chlorine Check Ultra High II bottle and set in a dry place, replace cap on bottle and tighten. Keep the test strip away from water until ready to test.
Step 2: Dip test strip into prepared solution sample for 1 full second.
Step 3: Remove strip from sample and shake the strip once, briskly, to remove excess solution sample (a trashcan is recommended). Caution: drops of disinfecting or sanitizing solution can bleach fabric and may cause skin and eye irritation.
Step 4: After 1 minute match the test pad to the closest color on the bottle color chart within the next 15 seconds. Results will show parts per million (ppm) concentration of Free Chlorine (bleach).
Guidelines for Disinfection and Sterilization from the CDC
Chlorine and Chlorine Compounds
Hypochlorites, the most widely used of the chlorine disinfectants, are available as liquid (e.g., sodium hypochlorite) or solid (e.g., calcium hypochlorite). The most prevalent chlorine products in the United States are aqueous solutions of 5.25%-6.15% sodium hypochlorite, usually called household bleach.
They have a broad spectrum of antimicrobial activity, do not leave toxic residues, are unaffected by water hardness, are inexpensive and fast acting, remove dried or fixed organisms and biofilms from surfaces, and have a low incidence of serious toxicity.
After reviewing environmental fate and ecologic data, EPA has determined the currently registered uses of hypochlorites will not result in unreasonable adverse effects to the environment.
Uses
Hypochlorites are widely used in healthcare facilities in a variety of settings. Inorganic chlorine solution is used for disinfecting tonometer heads and for spot-disinfection of countertops and floors. A 1:10-1:100 dilution of 5.25%-6.15% sodium hypochlorite (i.e., household bleach) or an EPA-registered tuberculocidal disinfectant has been recommended for decontaminating blood spills.
Chlorine has long been used as the disinfectant in water treatment. Hyperchlorination of a Legionella-contaminated hospital water system resulted in a dramatic decrease (from 30% to 1.5%) in the isolation of L. pneumophila from water outlets and a cessation of healthcare-associated Legionnaires' disease in an affected unit.
Hydrogen Peroxide
Hydrogen peroxide works by producing destructive hydroxyl free radicals that can attack membrane lipids, DNA, and other essential cell components. Catalase, produced by aerobic organisms and facultative anaerobes that possess cytochrome systems, can protect cells from metabolically produced hydrogen peroxide by degrading hydrogen peroxide to water and oxygen. This defense is overwhelmed by the concentrations used for disinfection.
Hydrogen peroxide is active against a wide range of microorganisms, including bacteria, yeasts, fungi, viruses, and spores. A 0.5% accelerated hydrogen peroxide demonstrated bactericidal and virucidal activity in 1 minute and mycobactericidal and fungicidal activity in 5 minutes.
Uses
Commercially available 3% hydrogen peroxide is a stable and effective disinfectant when used on inanimate surfaces. As with other chemical sterilants, dilution of the hydrogen peroxide must be monitored by regularly testing the minimum effective concentration (i.e., 7.5%-6.0%). Compatibility testing by Olympus America of the 7.5% hydrogen peroxide found both cosmetic changes (e.g., discoloration of black anodized metal finishes) and functional changes with the tested endoscopes (Olympus, written communication, October 15, 1999).
Peracetic Acid and Hydrogen Peroxide
Peracetic, or peroxyacetic, acid is characterized by rapid action against all microorganisms. Special advantages of peracetic acid are that it lacks harmful decomposition products (i.e., acetic acid, water, oxygen, hydrogen peroxide), enhances removal of organic material, and leaves no residue. It remains effective in the presence of organic matter and is sporicidal even at low temperatures (Tables 4 and 5). Peracetic acid can corrode copper, brass, bronze, plain steel, and galvanized iron but these effects can be reduced by additives and pH modifications. It is considered unstable, particularly when diluted; for example, a 1% solution loses half its strength through hydrolysis in 6 days, whereas 40% peracetic acid loses 1%-2% of its active ingredients per month.
Uses
The combination of peracetic acid and hydrogen peroxide has been used for disinfecting hemodialyzers. The percentage of dialysis centers using a peracetic acid-hydrogen peroxide-based disinfectant for reprocessing dialyzers increased from 5% in 1983 to 56% in 1997. FDA has cleared a newer chemical sterilant with 0.23% peracetic acid and 7.35% hydrogen peroxide. After testing the 7.35% hydrogen peroxide and 0.23% peracetic acid product, Olympus America concluded it was not compatible with the company's flexible gastrointestinal endoscopes; this conclusion was based on immersion studies where the test insertion tubes had failed because of swelling and loosening of the black polymer layer of the tube (Olympus America, personal communication, September 13, 2000).
Full Article
Cleaning refers to the removal of germs, dirt, and impurities from surfaces. Cleaning does not kill germs, but by removing them, it lowers their numbers and the risk of spreading infection.
Disinfecting refers to using chemicals to kill germs on surfaces. This process does not necessarily clean dirty surfaces or remove germs, but by killing germs on a surface after cleaning, it can further lower the risk of spreading infection.
Routine Cleaning
Community members can practice routine cleaning of frequently touched surfaces (for example: tables, doorknobs, light switches, handles, desks, toilets, faucets, sinks) with household cleaners and EPA-registered disinfectants that are appropriate for the surface, following label instructions. Labels contain instructions for safe and effective use of the cleaning product including precautions you should take when applying the product, such as wearing gloves and making sure you have good ventilation during use of the product.
How to Clean and Disinfect Surfaces (CDC)
- Wear disposable gloves when cleaning and disinfecting surfaces. Gloves should be discarded after each cleaning. If reusable gloves are used, those gloves should be dedicated for cleaning and disinfection of surfaces for COVID-19 and should not be used for other purposes. Consult the manufacturer's instructions for cleaning and disinfecting products used. Clean hands immediately after gloves are removed.
- If surfaces are dirty, they should be cleaned using a detergent or soap and water prior to disinfection
- For disinfection, diluted household bleach solutions, alcohol solutions with at least 70% alcohol, and most common EPA-registered household disinfectants should be effective.
- For soft (porous) surfaces such as carpeted floors, rugs, and drapes, remove visible contamination if present and clean with appropriate cleaners indicated for use on these surfaces.
Other Considerations (CDC)
- The ill person should eat/be fed in their room if possible. Non-disposable food service items used should be handled with gloves and washed with hot water or in a dishwasher. Clean hands after handling used food service items.
- If possible, dedicate a lined trash can for the ill person. Use gloves when removing garbage bags, handling, and disposing of trash. Wash hands after handling or disposing of trash.
- Consider consulting with your local health department about trash disposal guidance if available.



